40 phase labels in chemical equations
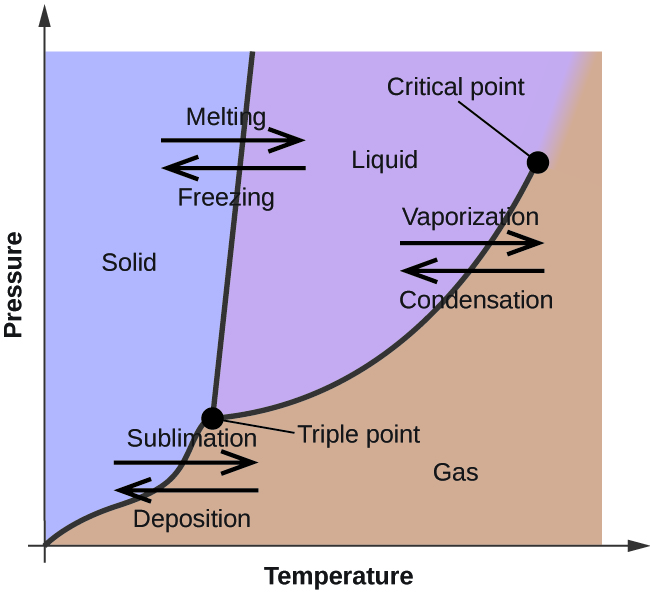
Phase Labels - YouTube Clark College Tutoring and Writing Center tutors Kevin Martin and Joey Smokey explain the usage of phase labels in chemical reactions such as (s), (l), (g), ... State symbols and phase changes | StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l)
Many chemical equations also include phase labels for Many chemical equations also include phase labels for the substances s for solid from AGRICULTUR 847,272 at Eastern Visayas State University - Tacloban City Main Campus

Phase labels in chemical equations
How do you write the phases of a chemical equation? How do you write the phases of a chemical equation? September 6, 2022 by Alexander. Solid, given the symbol (s) Liquid, given the symbol (l) Gas, given the symbol (g) Aqueous, meaning dissolved in water, and given the symbol (aq) READ SOMETHING ELSE. Table of Contents show 4.2: The Chemical Equation - Chemistry LibreTexts Exercise 4.2. 1. Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer. Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). The Chemical Equation | Introductory Chemistry - Lumen Learning In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products. ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as ...
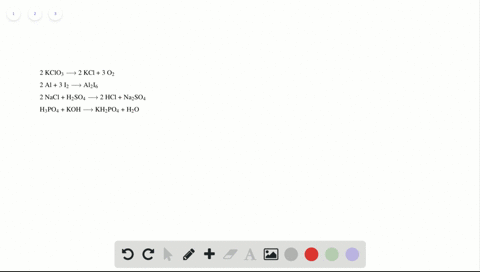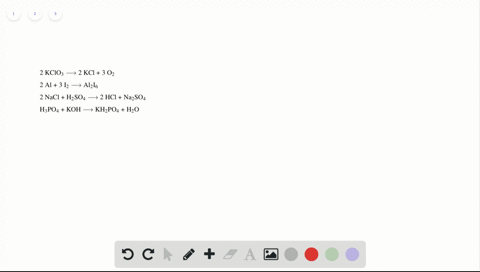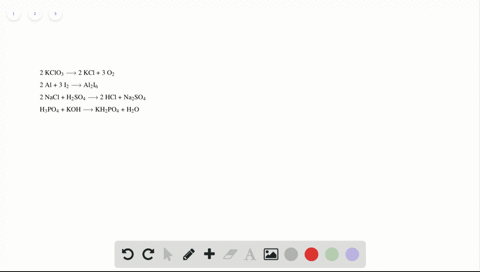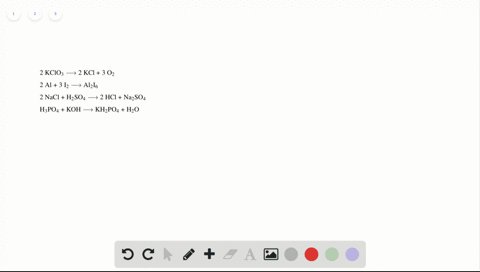
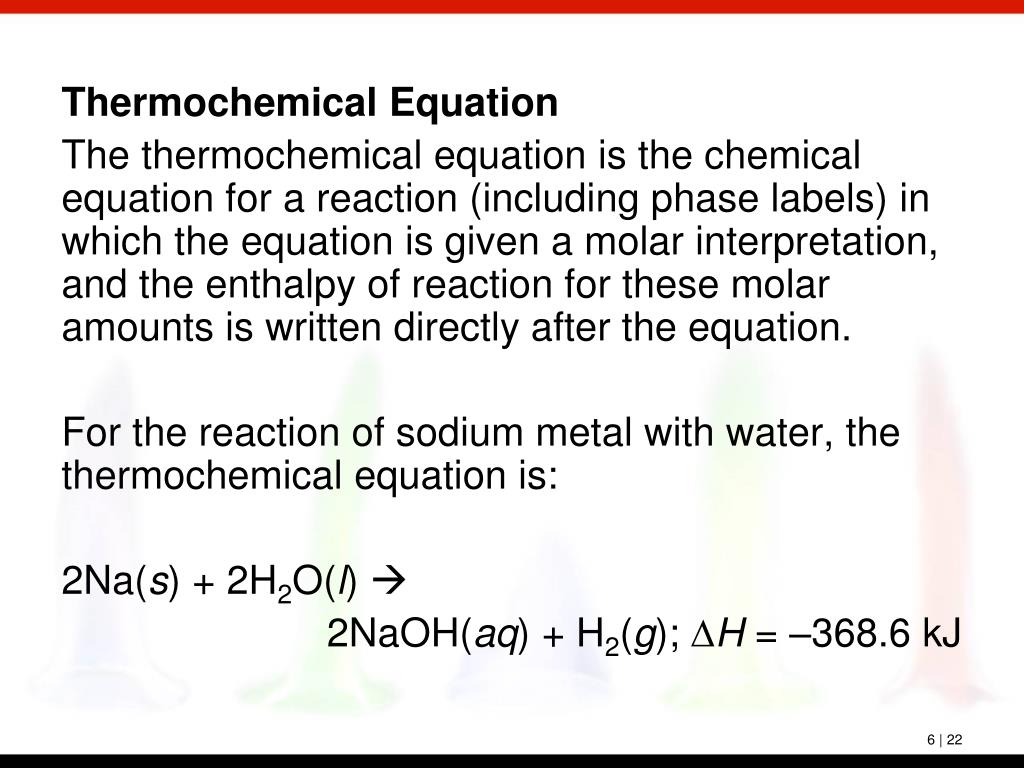
Phase labels in chemical equations. Solved 1. Write balanced chemical equations using proper | Chegg.com Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron(III) oxide, also known as rust. b) Solid calcium carbonate is added to a solution of hydrochloric acid, yielding an aqueous solution of calcium chloride, water, and carbon ... End-of-Chapter Material | Introductory Chemistry - Lumen Learning Write a chemical reaction for the boiling of water, including the proper phase labels. Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why. 4 Na(s) + 2 Cl 2 (g) → 4 NaCl(s) EXAM #2: Chapter 4 Flashcards | Quizlet Chemical equations can also be used to represent physical processes. Write a chemical reaction for the boiling of water, including the proper phase labels. ... SOLVED:16. Write a balanced equation (including phase labels) for the ... we are just looking to balance some chemical equations. So the first one we have a gcl in the solid state. I won't list. I went right down the states but I will say them aloud just to save a bit of times what a gcl solid at two and H. Three A quits. That gives us A G. And H. 32 plus acquis Art C. L minus liquids. The next example we've got C R E N two cl two cl acquits R two H 20.
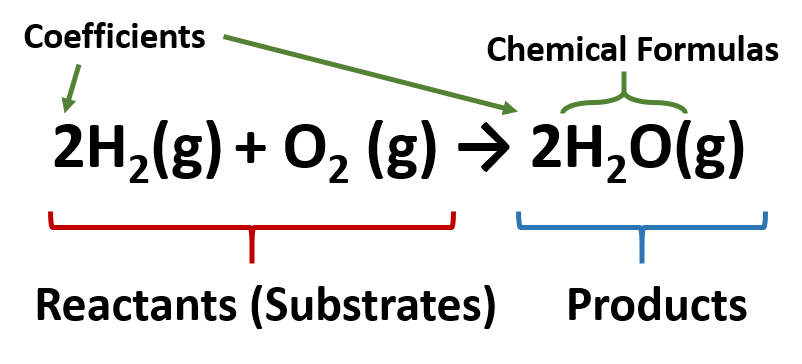
3 Steps for Balancing Chemical Equations - ThoughtCo A chemical equation describes what happens in a chemical reaction.The equation identifies the reactants (starting materials) and products (resulting substances), the formulas of the participants, the phases of the participants (solid, liquid, gas), the direction of the chemical reaction, and the amount of each substance. Chemical equations are balanced for mass and charge, meaning the number ... The Chemical Equation - Introductory Chemistry - 1st Canadian Edition In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products. ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as ... Definition of dissolve and the state label (aq) in chemical equations A colloid is a heterogeneous phase. But above all, do not worry too much about these minor symbolism issues. A chemical equation is not meant to convey everything. It is just a shorthand notation to describe chemical reactions. A real experiment or a research paper should describe the experimental condition with sufficient details in such a way ... End-of-Chapter Material - Introductory Chemistry - 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na(s) + 2Cl 2 (g) → 4NaCl(s) should not be considered a proper chemical equation. Explain why H 2 (g) + ½O 2 (g) → H 2 O(ℓ) should not be considered a proper chemical ...
Solved What is the outcome of the following reactions? Write | Chegg.com Expert Answer. 100% (1 rating) Transcribed image text: What is the outcome of the following reactions? Write balanced chemical equations including phase labels for any reaction that will produce a product. You may have to adjust the coefficients of the reactants to properly balance your reactions. (NH4)2 S(aq)+LiOH(aq)⋯ > Hgg(i)+Au(NO3)3 (aq ... 7.3: Chemical Equations - Chemistry LibreTexts Writing Chemical Equations. When sulfur dioxide is added to oxygen, sulfur trioxide is produced. Sulfur dioxide and oxygen, SO 2 + O 2, are reactants and sulfur trioxide, SO 3, is the product. 2SO 2(g) + O 2(g) ⏟ Reactants → 2SO 3(g) ⏟ Products. In chemical reactions, the reactants are found before the symbol " → " and the products are ... How do you label chemical equations? [FAQs!] The label on an original chemical container must be legible and written in English. It must include the chemical/product name as shown on the SDS and the. ... How do you label chemical equations? October 10, 2022 September 7, 2022 by Alexander. READ SOMETHING ELSE. Table of Contents show Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). ... phase label. To balance this equation, we need two phosphate ions and three calcium ions; we end up with six water molecules to balance the equation: 2H 3 PO 4 (aq) + 3Ca(OH) 2 ...
Chemical Reactions and Equations - GitHub Pages Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). ... According to the solubility rules, Ca 3 (PO 4) 2 is insoluble, so it has an (s) phase label. To balance this equation, we need two phosphate ions and three calcium ions; we end ...
The Chemical Equation | Introductory Chemistry - Lumen Learning In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products. ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as ...
4.2: The Chemical Equation - Chemistry LibreTexts Exercise 4.2. 1. Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer. Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water).
How do you write the phases of a chemical equation? How do you write the phases of a chemical equation? September 6, 2022 by Alexander. Solid, given the symbol (s) Liquid, given the symbol (l) Gas, given the symbol (g) Aqueous, meaning dissolved in water, and given the symbol (aq) READ SOMETHING ELSE. Table of Contents show

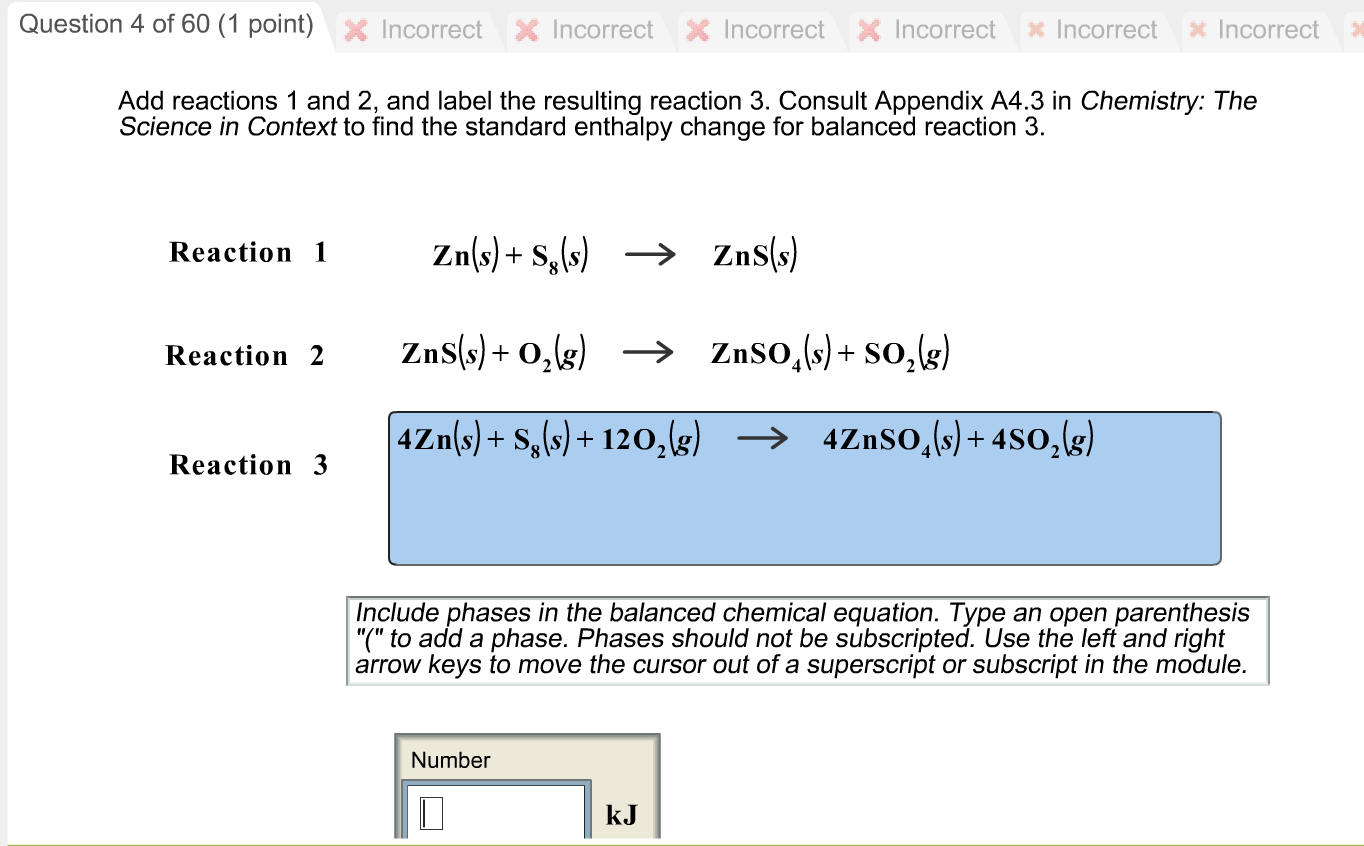
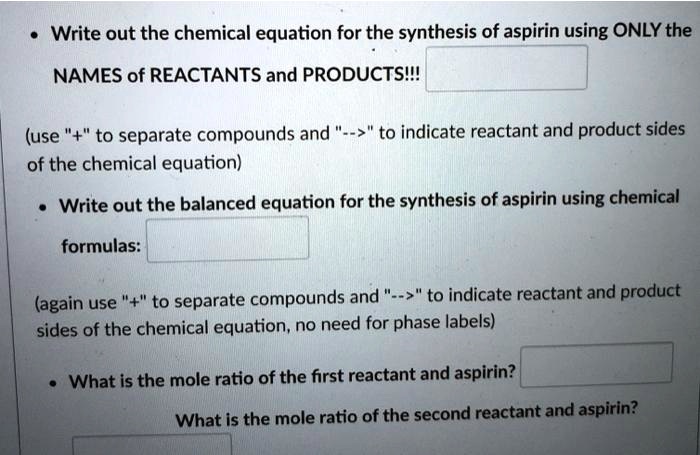
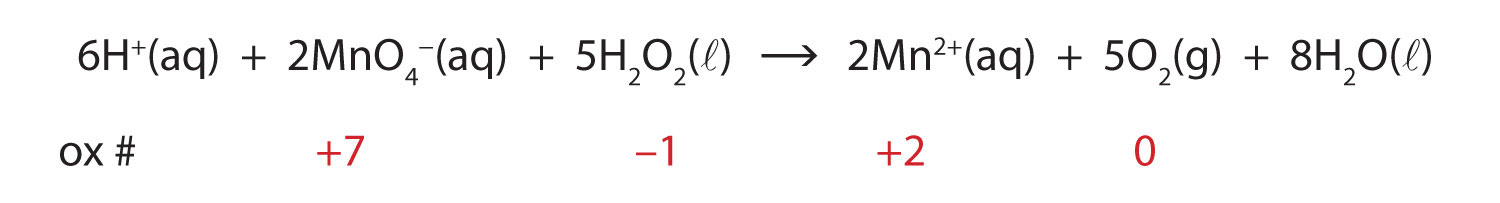



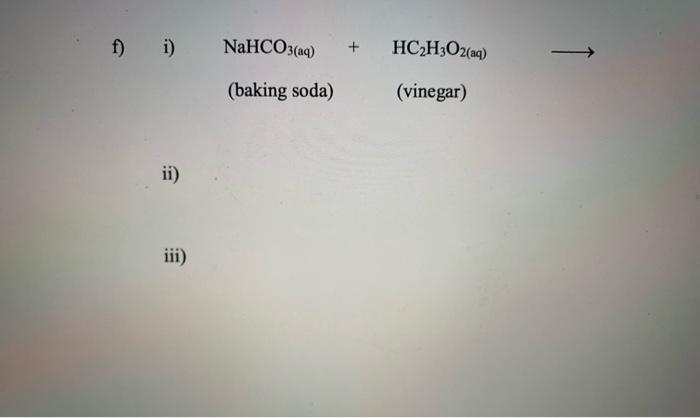











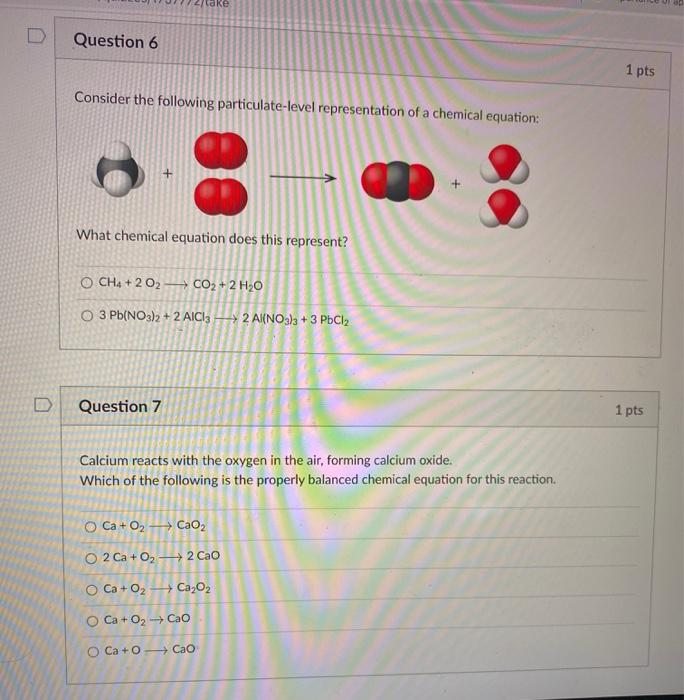
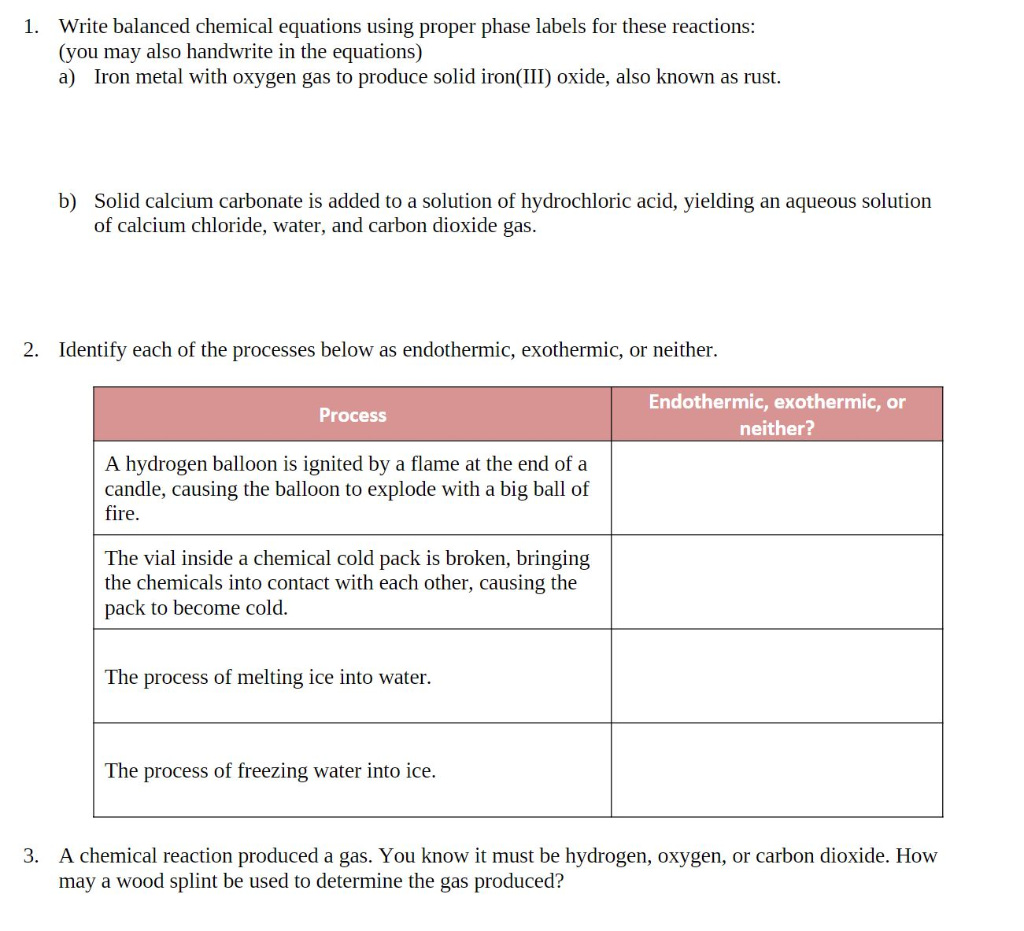





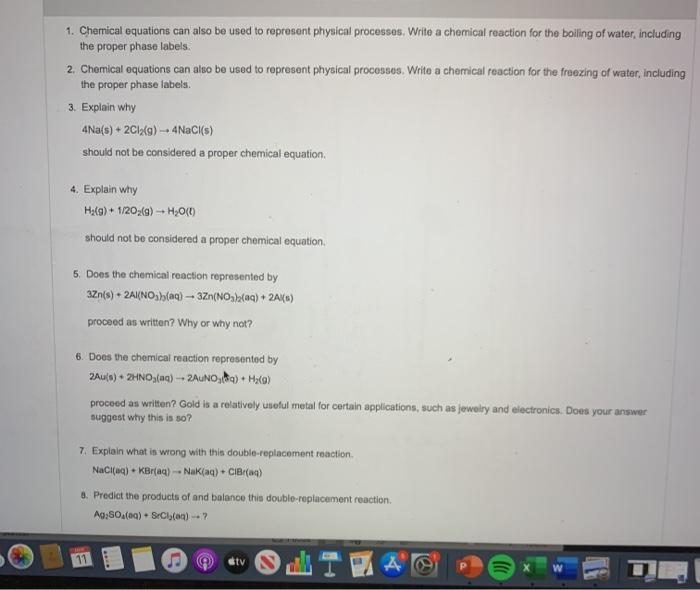


Post a Comment for "40 phase labels in chemical equations"